
-
Retinal absorbs photons*
-
Retinal changes shape
-
Retinal activates G-protein Transducin
-
T causes changes in cytoplasm concentration of secondary messengers
-
PDase converts cGMP to GMP
-
GMP gated ion channels are modulated
-
Changes in mmb pt
*The absorption of a photon also causes changes in opsin by activation of cGMP Phosphodiesterase
-
Most studied sense
-
There is never a static image because the eyes are always moving (saccadic pathway vs. smooth pursuit pathway)
-
120 Million rods, 6 million cones, in each eye
-
Rods are 1000 times more sensitive to light than cones
-
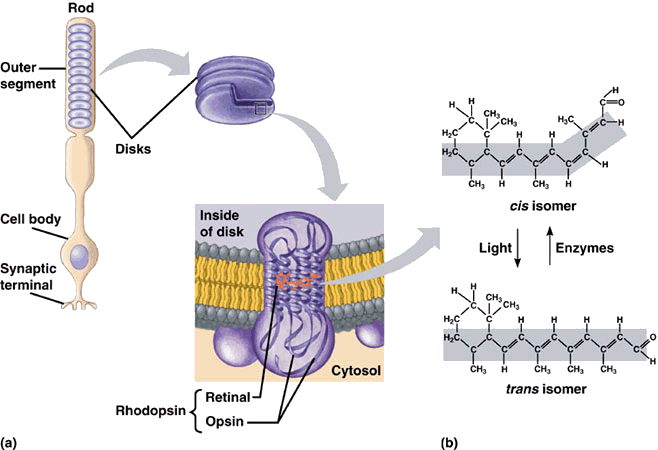
Rhodopsin (mmb protein) is found on disks of the outer segment of the photoreceptor
-
Retinal (aka Vitamin-A-Aldehyde)
-
Opsin- Ligand
-
Length of eyeball (adult) = 24.2 mm
-
Weight of eyeball = 7.5 g
-
Volume of eyeball = 5.5 cm^3
-
About 90% of these cells are very small, while another 5% are large M-type or magnocellular cells
-
Vision begins with light passing through the cornea, which does about three-quarters of the focusing, and then the lens, which adjusts the focus
-
The size of the pupil is controlled by the iris. The shape of the lens is altered by the muscles just behind the iris so that near or far objects can be brought into focus on the retina
-
Rods are extremely sensitive to light and allow us to see in dim light, but they do not convey color
-
Rods constitute 95% of all photoreceptors in humans
-
Most of our vision, however, comes from cones that work under most light conditions and are responsible for acute detail and color vision
-
Near the margins of the retina, each ganglion cell receives signals from many rods and cones, explaining why we cannot see fine details on either side
-
The retina contains three organized layers of neurons.
-
first layer (r&c) then
-
middle layer (interneurons), then
-
third layer, consisting of multiple different types of ganglion cells, specialized neurons near the inner surface of the retina
VISION


Perception of movement, depth, perspective, the relative size of objects, the relative movement of objects, shading, and gradations in texture all depend primarily on contrasts in light intensity rather than on color.
-
three separate processing systems:
-
One appears to process information mainly about shape
-
second, mainly about color
-
third, movement, location, and spatial organization.
-
The axons of the ganglion cells form the optic nerve
-
Each neuron in the middle and third layer typically receives input from many cells in the previous layer, and the number of inputs varies widely across the retina. Near the center of the gaze, where visual acuity is highest, each ganglion cell receives inputs — via the middle layer — from one cone or, at most, a few, allowing us to resolve very fine details
How it works: Phototransduction
(in dark)
-
Photon converted to chemical signal in Outer segment
-
Chemical signal converted to electrical signal in Outer terminal
-
Electrical signal converted to chemical signal in Inner segment
Phtotransduction In light
About 60 years ago, scientists discovered that each vision cell’s receptive field is activated when light hits a tiny region in the center of the field and inhibited when light hits the area surrounding the center. If light covers the entire receptive field, the cell responds weakly.
the visual process begins by comparing the amount of light striking any small region of the retina with the amount of surrounding light.
primary visual cortex — a thin sheet of tissue (less than one-tenth of an inch thick)
-
A light photon interacts with the retinal in a photoreceptor cell. The retinal undergoes isomerisation, changing from the 11-cis to all-trans configuration
-
Retinal no longer fits into the opsin binding site.
-
Opsin therefore undergoes a conformational change to metarhodopsin II.
-
Metarhodopsin II is unstable and splits, yielding opsin and all-trans retinal.
-
The opsin activates the regulatory protein transducin. This causes transducin to dissociate from its bound GDP, and bind GTP, then the alpha subunit of transducin dissociates from the beta and gamma subunits, with the GTP still bound to the alpha subunit.
-
The alpha subunit-GTP complex activates phosphodiesterase or PDE.
-
PDE breaks down cGMP to 5'-GMP. This lowers the concentration of cGMP and therefore the sodium channels close.
-
Closure of the sodium channels causes hyperpolarization of the cell due to the ongoing efflux of potassium ions.
-
Hyperpolarization of the cell causes voltage-gated calcium channels to close.
-
As the calcium level in the photoreceptor cell drops, the amount of the neurotransmitter glutamate that is released by the cell also drops. This is because calcium is required for the glutamate-containing vesicles to fuse with cell membrane and release their contents.
-
A decrease in the amount of glutamate released by the photoreceptors causes depolarization of On center bipolar cells (rod and cone On bipolar cells) and hyperpolarization of cone off-center bipolar cells.

Illuminations effects on Mmb ptns
Dark
Light
RODS CONES
-
cGMP levels are high and keep cGMP-gated sodium channels open allowing a steady inward current, called the dark current. This dark current keeps the cell depolarized at about -40 mV.
-
The depolarization of the cell membrane in scotopic conditions opens voltage-gated calcium channels.
-
An increased intracellular concentration of Ca2+ causes exocytosis.
In the cone pathway glutamate:
-
Hyperpolarizes on-center bipolar cells. Glutamate that is released from the photoreceptors in the dark binds to metabotropic glutamate receptors (mGluR6), which, through a G-protein coupling mechanism, causes non-specific cation channels in the cells to close, thus hyperpolarizing the bipolar cell.
-
Depolarizes off-center bipolar cells. Binding of glutamate to ionotropic glutamate receptors results in an inward cation current that depolarizes the bipolar cell.
-
Photoreceptors hyperpolarize to a potential of -60mV. It is this 'switching off' that activates the next cell and sends an excitatory signal down the neural pathway.
-
Depletes cGMP in Cones
-
Small cones
-
β-type
-
2% of 6-7 Million
-
outside the fovea (greatest resolution
-
More sensitive to light than L or M
-
the refractive indexfor blue light is enough different from red and green that when they are in focus, the blue is slightly out of focus (chromatic aberration).
CONES
-
Medium cones
-
γ-type
-
32% of 6-7 Million
-
-
Large cones
-
ρ-type
-
64% of 6-7 Million
-
3 Types
RED
GREEN
BLUE



-
Sensitivity to a prolonged stimulation tends to decline over time, leading to neural adaptation. An interesting effect occurs when staring at a particular color for a minute or so. Such action leads to an exhaustion of the cone cells that respond to that color - resulting in the afterimage. This vivid color aftereffect can last for a minute or more.
-
If overexposed to one color of light, the pigments in the cones adapt and then make a lesser contribution to our perception of color for a short while thereafter.
-
Death of photoreceptors in the macula, called macular degeneration, is a leading cause of blindness among the elderly population in developed countries, including the United States
-
Green and red cones are concentrated in the fovea centralis (0.3 mm diameter) (macula)
-
The "blue" cones have the highest light sensitivity and are mostly found outside the fovea, leading to some distinctions in the eye's blue perception
-
Like all neurons, the cones fire to produce an electrical impulse on the nerve fiber and then must reset to fire again. The light adaption is thought to occur by adjusting this reset time
-
However, the blue sensitivity of our final visual perception is comparable to that of red and green, suggesting that there is a somewhat selective "blue amplifier" somewhere in the visual processing in the brain
-
Protanopia, deuteranopia, protanomaly, and deuteranomaly are commonly inherited forms of red-green color blindness
-
Those with tritanopia and tritanomaly have difficulty discriminating between bluish and greenish hues, as well as yellowish and reddish hues
-
Color blindness involving the inactivation of the short-wavelength sensitive cone system (whose absorption spectrum peaks in the bluish-violet) is called tritanopia
-
The disorder called ocular motor apraxia, in which the saccadic pathway doesn't function. These people (usually children that grow out of it) rely entirely on their pursuit pathway. Once they fixate on an object, they can't look away unless they turn their heads so far as to force their eyes to refixate.
-
genetic basis of color blindness is due to:
1. the absence of certain visual pigments,
2. the function of the retinal network
3. the presence of two different types of ganglion cells.
Color vision table
Name of state ~number of colors perceived Porters
Monochromacy: 100 marine mammals, owl monkey, Australian sea lion, achromat
Dichromacy: 10,000 most terrestrial non-primate mammals, color blind primates
Trichromacy: 10 million most primates, espec. great apes, marsupials,some insects (such as honeybees)
Tetrachromacy: 100 million most reptiles, amphibians, birds and insects, rarely humans
Pentachromacy: 10 billion some insects (specific species of butterflies), some birds (pigeons for instance)

Cones and Light Wavelengths
-
Reach peak sensitivity between small and medium wavelengths
-
Photopsin (AKA conopsin)
-
has difference amino acids for S,M,L cones
-
Similar function to rhodopsin
-
Activated by light
-
The opsins (photopigments) present in the L and M cones are encoded on the X chromosome; defective encoding of these leads to the two most common forms of color blindness.
-
A very small percentage of women may have an extra type of color receptor because they have different alleles for the gene for the L opsin on each X chromosome. X chromosome inactivation means that only one opsin is expressed in each cone cell, and some women may therefore show a degree of tetrachromatic color vision.
-
cones adapt much faster to chamges in illumination compared to rods
Retinofugal Projections
The neural pathways that leave the eye collectively referred to as the retinofugal pathway, which can be translated from Latin as fleeing the retina.
Tectum=superior colliculi=retinotectal regions=orienting to stimuli in environment/visual reflexes
Involuntary visual reflexes are controlled by the accessory optic system known as optokinetic reflex
Other retinal axons go to other smaller nuclei in the brain, such as the accessory optic system. These nuclei are involved in involuntary reflexes, such as the optokinetic reflex we mentioned earlier.
There are 5 major stops before the striate cortex
-
Optic Nerve
-
Optic Chiasm
-
Optic Tract
-
LGN
-
Optic radiation
LGN
AKA dorsolateral Thalamus
Left LGN, Left VF. Right LGN, Right VF.
6 layers of LGN, 3 per each side
Information from the contralateral side goes to layers 1,4,6 while
ipsilateral goes to 2,3,5
2 Ventral Layers: larger neurons- Magnocellular (to dorsal pathway)
4 Dorsal Layers: smaller neurons- Parvocellular (to ventral pathway)
Neurons in between: Koniocellular (color information)
Magnocellular
-
Large centre surround RF
-
no color
-
quick, transient bursts of firing in response to moving objects
Parvocellular
-
Small centre surround RF
-
sustained firing in response to object shapes
-
feature discrimination
-
boundaries of object
-
poor temporal resolution (cannot track if moving)
-
color, tecture, depth
Koniocellular
-
color information
80% of visual synapses are returning synapses from the VC
Feedback activity through thalamocortical loops hosts prominent rhythmic brain waves (EEG)
Strabismus: Beyond the age of 8 or so, the blindness in one eye becomes permanent.
Recently, gene therapy for a small group of patients with severe blindness allowed them to see. Work also is in progress to bypass lost photoreceptors and send electrical signals directly to the brain via ganglion cells.



Striate
-
6 Cortices in most areas
-
2 mm dark matter
Ocular dominance column: striped pattern of Neurons that respond to left or right eye
Covering either of the eyes would cause death of the respective neurons in the cortical columns
In PV1 middle layer, which receives messages from the lateral geniculate nucleus, scientists have found responses similar to those seen in the retina and in lateral geniculate cells.
Layers of pvc




Lesions of the Visual cortex
Ventral pathway
V1 V2 V4 IT
RF size |-->-->-Increases->->->->
Complexity ⋅ \|/ LT SCO 👲 👳
Conclusion: RF and sensitivity to complex stimuli increases
Damage
V1/V2: Scotoma (blindspot) BC its organized retinotopically ( 1 direction corresponds to 1 direction of visual space)
V4/IT: Unrecognition of objects or faces: AGNOSIA or PROSOPAGNOSIA
Damage to V1 may result in Blindsight, which is mediated by parallel connections from the eyes to other parts of the cortex.
Dorsal pathway
MT (midtemporal/ V5)
-
direction of movement
-
damage= motion blindnesss (sees series of snapshots)
-
V5 responds selectively to particular directions of movement, increasing activity systematically and accurately when the proportion of stimuli moving in the preferred motion direction increases
-
also registers perceived motion
-
If the movement is completely random, neurons that normally prefer rightwards movement fire slightly more on trials when the observer reports that the random motion signal is moving “rightwards” (and vice versa)
-
The difference between neuronal decisions of “rightwards” or “leftwards” reflects what the observer judges about the appearance of motion, not the absolute nature of the moving stimulus
-
Other examples of visual decision and indecision include reactions to perceptual targets that are genuinely ambiguous, such as the so-called Necker cube
-
The resulting percept is termed binocular rivalry, as the observer reports first that the vertical lines dominate, then the horizontal lines and then back again to vertical
-
Perception of movement, depth, perspective, the relative size of objects, the relative movement of objects, shading, and gradations in texture all depend primarily on contrasts in light intensity rather than on color.
PPA
-
directing attention to particular parts of space in charge of spatial attention
-
figure-ground seperation
-
damage= apraxia, hemispatial neglect (unilateral damage, cannot pay attenion to 50% RF or 50% body feels foreign) , visual neglect
Hemianopia Vascular and neoplastic (malignant or benign tumours) lesions from the optic tract, to visual cortex can cause a contralateral homonymous hemianopsia. Injury to the right side of the brain will affect the left visual fields of each eye. The more posterior the cerebral lesion, the more symmetric (congruous) the homonymous hemianopsia will be. For example, a person who has a lesion of the right optic tract will no longer see objects on his left side. Similarly, a person who has a stroke to the right occipital lobe will have the same visual field defect, usually more congruent between the two eyes, and there may be macular sparing. A stroke on the right side of the brain (especially parietal lobe), in addition to producing a homonymous hemianopsia, may also lead to the syndrome of hemispatial neglect.
Damage to PPA and MT causes
-
Balint syndrome
several primary symptoms including
-
simultanagnosia (impaired spatial awareness of more than one object at time, inability to: experience world as a whole// pay attention anywhere in the VF),
-
optic ataxia (misreaching to visual targets),
-
ocular apraxia (described by Bálint as “psychic paralysis of gaze”) and general visuospatial disorientation



Left: positive absolute scotoma
Right: scintillating scotoma









Retinotopy is the mapping of visual input from the retina to neurons, particularly those neurons within the visual stream. For clarity, 'retinotopy' can be replaced with 'retinal mapping', and 'retinotopic' with 'retinally mapped'.




Confusing terms:
apraxia: inability to do purposive movements
ataxia: the loss of full control of bodily movements.
aphasia: avoir le mot sur la bout de la langue
agnosia: inability to recognize objects or faces
anosmia: inability to smell
anomia: tip of the tongue
anasognosia: inability to realize one is disabled
Anoxic brain damage is injury to the brain due to a lack of oxygen.
Hypoxia is the term to describe low oxygen.



